We’re proud to introduce our Evidence Speaks series, a recurring feature highlighting the latest in CHÉOS research. This series features summaries of select publications as well as in-depth features on the latest work from our investigators. In the early days of CHÉOS, the Centre had a series known as “The Evidence Speaks,” a monograph series to keep media and the research community up-to-date with CHÉOS’ current research results in the health outcomes field.
Oviedo-Joekes E, Brissette S, MacDonald S, Guh D, Marchand K, Jutha S, Harrison S, Janmohamed A, Zhang DZ, Anis AH, Krausz M, Marsh DC, Schechter MT. Safety profile of injectable hydromorphone and diacetylmorphine for long-term severe opioid use disorder. Drug Alcohol Depend. 2017 May 10;176:55-62.
Palis H, Marchand K, Guh D, Brissette S, Lock K, MacDonald S, Harrison S, Anis AH, Krausz M, Marsh DC, Schechter MT, Oviedo-Joekes E. Men’s and women’s response to treatment and perceptions of outcomes in a randomized controlled trial of injectable opioid assisted treatment for severe opioid use disorder. Subst Abuse Treat Prev Policy. 2017 May 19;12(1):25.
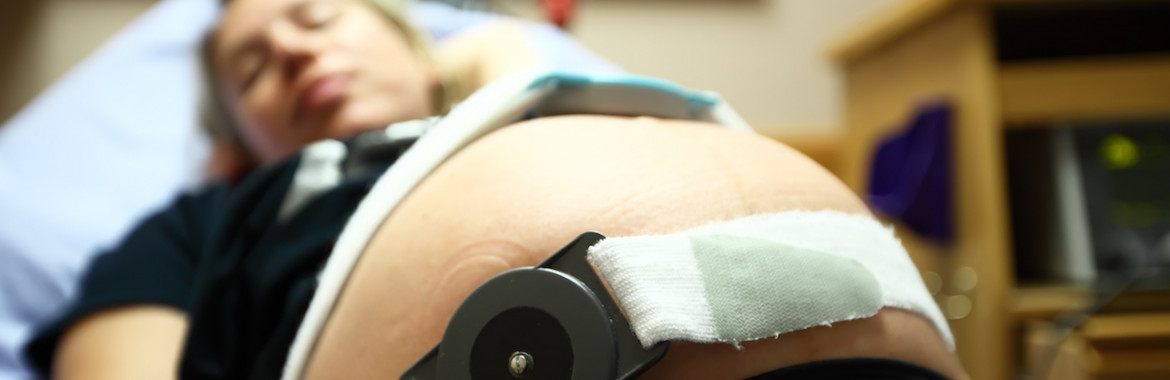
Two papers on secondary analyses of the Study to Assess Longer Term Opioid Medication Effectiveness (SALOME) trial were published in May. The SALOME trial found that hydromorphone, a legally available prescription opioid, was as effective as diacetylmorphine (medical heroin) in treating long-term opioid addiction in individuals for whom other treatments, like methadone, were not effective. One secondary analysis, co-authored by CHÉOS Scientists Drs. Eugenia Oviedo-Joekes, Aslam Anis, Michael Krausz, and Martin Schechter, analyzed the trial results by gender. The analysis found that there were no significant differences in treatment outcomes (six months) and trial retention between men and women. Women were significantly more likely to be involved in sex work than men, both at baseline and six months, and engagement in sex work was not changed significantly by trial participation. While women had poorer psychological health at baseline, it was significantly better compared to men after six months.
The second publication focused on the patterns of adverse events seen during the SALOME trial. Over the 6-month study period, participants experienced a total of twenty-nine serious adverse events, including fourteen opioid overdoses. These events were treated and resolved on site by medical response. The diacetylmorphine group had significantly higher rates of post-injection reactions and life threatening opioid overdoses compared to the hydromorphone group. There was no significant relationship found between total number of treatment days or daily dose and related adverse events for either treatment. Both secondary analyses highlight the effectiveness of medically prescribed and supervised injectable opioids and the value of maintaining engagement with the health care system in this population.
—
Polisena J, Burgess M, Mitton C, Lynd LD. Engaging the Canadian public on reimbursement decision-making for drugs for rare diseases: a national online survey. BMC Health Serv Res. 2017 May 26;17(1):372.
What are Canadian’s priorities and perspectives related to the funding of expensive drugs for rare diseases? To answer this question, CHÉOS Scientist Dr. Larry Lynd led a national online survey to engage a wide variety of people across Canada. Sixteen-hundred people from across Canada completed the survey. Respondents chose and ranked, by importance, up to five decision-making priorities out of a list of eight potential items. Respondents also rated their agreement with four funding scenarios and were able to view how each scenario interacted with their selected priorities. Results showed that improved quality of life and effective health care were top priorities to be considered when making decisions about funding for rare diseases drugs. Generally, respondents felt that all Canadians should have equal access to the same drugs and that a drug should be funded if justified by its cost effectiveness. Half of respondents felt that drugs for rare diseases should be funded regardless of the cost. From the perspective of a decision-maker, these results may be important for policy decision and implementation.
—
Li LC, Shaw C, Lacaille D, Yacyshyn E, Jones CA, Koehn C, Hoens AM, Geldman J, Sayre EC, Macdonald G, Leese J, Bansback N. Effects of a web-based patient decision aid on biologic and small molecule agents for rheumatoid arthritis, ANSWER-2: A proof-of-concept study. Arthritis Care Res. 2017 May 23 epub ahead of print.
Patient engagement in decision making about treatment options is an important and increasingly popular part of clinical practice. The addition of a biologic to a treatment regimen for people with rheumatoid arthritis (RA) can improve treatment outcomes in some patients but also increases the risk of adverse events. CHÉOS Scientists Drs. Linda Li and Nick Bansback, along with Knowledge Broker Alison Hoens, studied the effect of an online patient decision aid on patient decision making, knowledge, and self-management. The study employed the recently developed, web-based ANSWER-2 decision aid. In an individualized setting, this tool summarizes the benefits and risks of staying on a current regimen, modifying a current regimen, or switching to a new one. Users also rate the importance of attributes of biologic agents including administration, amount of evidence, and risk of side effects. Through a series of questionnaires and an in-depth interview, the research team found that, in general, the ANSWER-2 improved the quality of decision making and perceived self-management in study participants. The findings also suggest that decision aids alone may be not be effective in some people and that experiences with the tool may be shaped by a participant’s relationship with their physician. Two trials are underway to compare ANSWER-2 to online educational material for people with RA.