Last year, in Part 1 of our series on rare diseases, we published a story about the Purcells and the challenges faced by families affected by rare diseases, such as accessing genetic services and the cost of treatment. In part two of this series, we are exploring other ongoing research led by Advancing Health Scientist Dr. Larry Lynd, which is focused on what the future could look like for the diagnosis and treatment of these diseases.
—
Dr. Lynd notes that one of the biggest issues faced by families across the world who are affected by rare diseases, defined as any disease that affects less than 1 in 2000 people, is access to genetic services and diagnosis.
In B.C., the wait time to see a genetic counsellor or clinical geneticists can be two years or more. This wait can be excruciating for families as they try to find other specialists who can help them. A diagnosis can help families access other programs, funding, and services, even if there isn’t a treatment available for the disease they are dealing with.

On top of the challenging wait times in B.C., Dr. Lynd notes that many of the genetic services are concentrated in the Lower Mainland, adding an extra barrier to people who live in other parts of the province.
To address these issues, Dr. Lynd and his team are conducting a series of studies to understand future needs and test innovative methods to improve access to genetic services.
Electronic tools help streamline access to services
In DECIDE, a project led by Dr. Lynd and Advancing Health’s Program Head of Decision Sciences Dr. Nick Bansback, researchers are developing and testing the use of electronic tools to improve accessibility and help ease the burden on genetic services. These efforts include an online decision aid to help patients make choices about genome testing that align with their values, as well as a telehealth approach and alternative ways of providing genetic counselling.
“With DECIDE, we’re looking at giving people ways of accessing services earlier, which for some people, could change their diagnostic trajectory and an unnecessarily long time on a waitlist,” Dr. Lynd explains.
Innovative approaches that can manage the burden on the genetics workforce are vitally important, says Dr. Lynd, because demand for these services has been steadily increasing — a trend that is expected to continue.
Managing demand: Increasing capacity and predicting future needs
Without access to genetic services, families like the Purcells would be unable to find a solution to their loved one’s problems; in their case, a rare genetic disorder affecting the metabolism called Hunter syndrome. The popularity of these services is growing rapidly due to technological advancements, increased awareness, and the fact that genetic information is becoming more clinically useful.
But this increased demand is outstripping the capacity of the genetic workforce in Canada. “There are about 450 genetic counsellors and 115 clinical geneticists for the whole country,” notes Dr. Lynd. “We graduate only 26 people per year, not all of whom go into clinical practice.”
Dr. Lynd and his team have been conducting a series of modelling studies to understand the current workforce requirements and estimate the number of clinical genetic service providers that will be needed in the future for things like hereditary cancer, genetic disorders, and prenatal testing and screening.
Beyond these genetic health care providers, genetic testing requires lab personnel and equipment to sequence the genome and a bioinformatician to extract the appropriate data from the genome and provide that information to the health care providers. These staffing requirements further compound the issues affecting accessibility to genetic diagnostic services.
Preliminary findings presented to the Canadian Agency for Drugs and Technologies in Health (CADTH) showed that, by 2030, we will need an additional 250 genetic counsellors just to meet the need for services in hereditary cancer alone, if there are no significant changes to service delivery models. This modelling could be used to inform workforce planning and investment in personnel, training, and other infrastructure, with the goal of increasing equitable access to genetic services for Canadians.
Looking forward: Curative therapy
More than 80 per cent of rare diseases have a single-gene cause, meaning that there is a possibility that some of these illnesses could be cured by one successful gene therapy intervention.
Gene therapies work by modifying the genetic anomaly responsible for the disease. This is in contrast to many of the drugs used for chronic conditions, which work to minimize symptoms rather than offer a cure.
“Gene therapies are expensive, but they are theoretically one and done,” explains Dr. Lynd. “This means that you could end up avoiding a lifetime of expensive treatments and specialist visits.”
In a paper published last year, Dr. Lynd and his team reviewed the economic evidence behind the use of genetic therapy products. They found that gene therapies are often more effective than standard treatments, but their cost rendered some of them uneconomical.
Related to this, Dr. Lynd and his team have recently received funding from the Nanomedicines Innovation Network to support the development of an early health technology assessment platform, which will include the early evaluation of new nanotechnologies for the delivery of gene therapies for rare diseases.
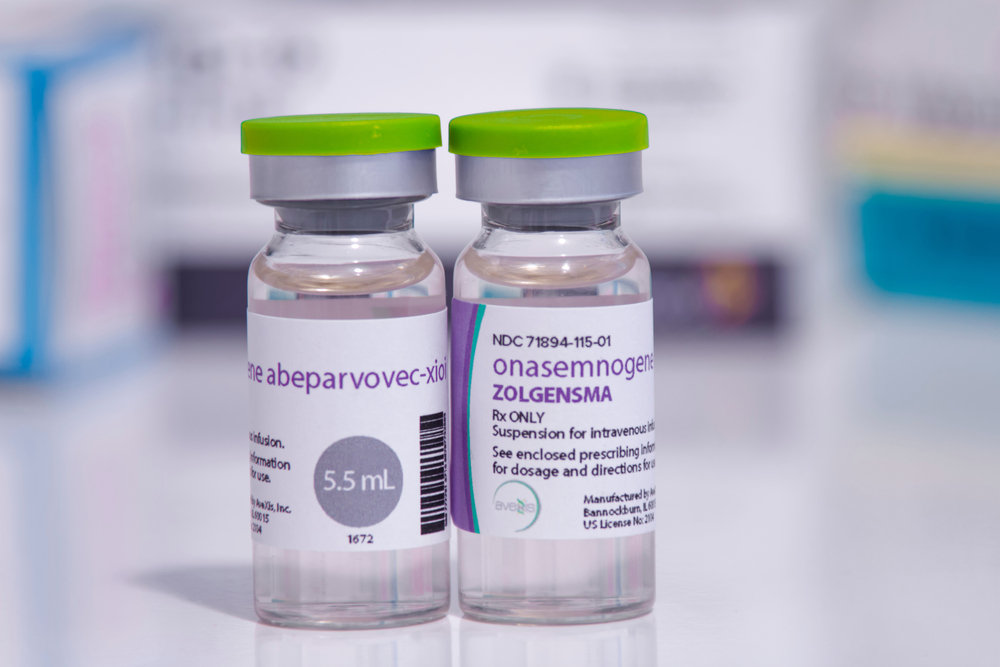
An example of one of these therapies is onasemnogene abeparvovec (Zolgensma®), a gene therapy that may cure spinal muscular atrophy type 1 (SMA-1). It was approved by Health Canada in 2020 at a list price of $2.9M per patient. SMA-1 is a very rare disease, but treatment costs of this magnitude pose serious challenges for a public health care system like Canada’s.
Despite their high cost, Dr. Lynd says that some gene therapies that aim to cure serious conditions that cause high rates of mortality and morbidity could be cost-effective compared with standard treatments.
“The issue with gene therapies is that we don’t yet know how efficacious and cost-effective they are in the long term; whether they offer a permanent cure,” Dr. Lynd expressed. “What we do know is this is a very exciting and emerging area in the treatment of rare diseases.”
But this uncertainty, says Dr. Lynd, both about the future of rare disease and their treatments, means that we need more research to help inform these difficult decisions moving forward.