More than a year since COVID-19 first emerged, there are still no proven effective treatments. While there are at least three widely approved COVID-19 vaccines already saving lives, clinicians and researchers have trialed and tested several drugs and therapies that initially seemed promising — including hydroxychloroquine and ivermectin — without any significant breakthroughs. Some treatments haven’t even worked at all.
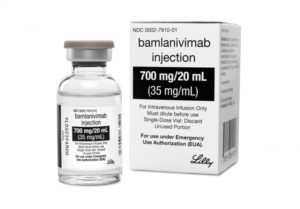
But a new human antibody identified by Vancouver biotech company AbCellera is showing early positive results. Bamlanivimab was recently approved by Health Canada and the US Food and Drug Administration for interim emergency use for the treatment of COVID-19. Supported by CHÉOS research services, Dr. Greg Haljan, the head of critical care at Surrey Memorial Hospital, will be launching a clinical study within two months in the Fraser Health area to test its effectiveness.
Working overtime to get study running
The timeline for this $1–million study is extremely aggressive, according to the CHÉOS Biostatistics Program Head Dr. Hubert Wong. CHÉOS is providing support to the B-EPIC study, as it’s called. “Typically, all the work to set up a clinical trial takes place over a year or more, but for B-EPIC, it has to be done within a couple of months.”
The CHÉOS team has several people involved in B-EPIC, explains Erin Cherban, Chief Clinical Research Officer at CHÉOS. “The first part is getting project managers in place to actually operationalize the study. Then we have to finalize the budget with the study team, help set up a database for study data, get contracts signed. Essentially, we have to figure out all those little pieces, coordinate with the right partners and people, especially the health authority operational team who is running the treatment clinics, and make everything and everyone work together.”

Dr. Wong is helping with the design of the trial, which determines when and how many patients will receive bamlanivimab. He is also overseeing the analysis of the data. In addition, CHÉOS will provide oversight for the Data Safety Monitoring Committee, which will keep track of the safety of participants. It all adds up to hundreds, if not thousands, of hours of work.
Logistics of study are challenging
Dr. Haljan and his team are planning to recruit more than 500 people 65 years of age and older who have COVID-19, though with the upcoming vaccination schedule, the age limit may be lowered. Their hope is treatment with bamlanivimab, which is a one-hour intravenous infusion, will reduce the likelihood of developing severe disease and requiring hospital care.
The treatment locations have to be isolated from high-traffic areas as all the patients will be COVID-19 positive. Cherban says even organizing the logistics around that are challenging, but she adds, “Everyone who is involved in B-EPIC has jumped in and is really collaborative. Usually, it’s more competitive, but COVID-19 has everyone working together.”

Research in pandemic times rewarding
“It’s rewarding to be doing something that can make a big difference,” says Dr. Wong. “Many of the negative impacts of the pandemic are emotionally distressing because you feel helpless against stopping them.”
Cherban agrees, especially because of the personal impact of the pandemic on her family. Her husband has heart failure and is at high risk of serious illness, so their two sons haven’t returned to in-class learning to protect their father. They’ve been socially isolating at home for nearly a year now.
Cherban feels optimistic about this new drug, and also about the future of research post-pandemic. She says, “A lot of COVID-19 clinical trials have really pushed the envelope. They’ve shown that we can streamline a lot of the administrative procedures that have traditionally slowed down the pace of research. Every study we do now, including B-EPIC, is also a study of our regulations. I hope it’s the catalyst for permanent change in our world of research.”

More about Banlanivimab
(excerpted from AbCellera news release)
Bamlanivimab was developed from an antibody that was discovered from the blood of a recovered COVID-19 patient using AbCellera’s pandemic response platform, in partnership with the Vaccine Research Center (VRC) at National Institute of Allergy and Infectious Diseases (NIAID). Within one week of receiving the sample, AbCellera screened over five million antibody-producing cells to identify and isolate approximately 500 unique antibodies that bind to SARS-CoV-2, the virus that causes COVID-19. The binding antibodies were then tested by AbCellera, the VRC, and Eli Lilly and Company to find those most effective in neutralizing the virus – essentially blocking the virus from attaching and entering human cells. Bamlanivimab was selected as the lead candidate from this group of antibodies, and was the first therapeutic candidate specifically developed against SARS-CoV-2 to enter human clinical trials in North America.